A supercapacitor (also called a supercap, electrochemical capacitor, ultracapacitor or Goldcap) is a high-capacity capacitor with higher capacitance values but lower voltage limit much than other capacitors that bridge the gap between electrolytic capacitors and rechargeable batteries. It is an electrochemical element developed in the 1970s ~1980s to store energy through polarized electrolytes. They typically store 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerate many more charge and discharge cycles than rechargeable batteries.
It is different from the traditional chemical power supply, and is a power supply which has special performance between the traditional capacitor and the battery, and mainly relies on the electrostatic double- layer and the oxidation-reduction pseudocapacitance to store electric energy. But there is no chemical reaction in the process of its energy storage, and this type of energy storage is reversible.
Supercapacitor, as a new type of energy storage device, which has the advantages of fast charging and discharging speed, high efficiency, good stability, long service life and so on, and is a clean green energy source, which is a new type of green energy in the 21st century, it has great market potential.

Supercapacitor Basics
1) Structure
The structure details of supercapacitors depend on the application of it. In other words, these materials may vary slightly relying on the manufacturer or specific application requirements. All supercapacitors have basic common points: they contain a positive electrode, a negative electrode, and a diaphragm between the two electrodes, and the electrolyte fills in the two pores separated from the two electrodes and the diaphragm.
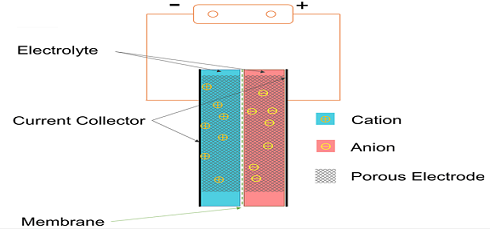
The structure of the supercapacitor is as shown in the following, which is composed of a porous electrode material, a current collector, a porous battery separator and an electrolyte. The electrode material is closely connected to the current collector to reduce contact resistance; the diaphragm shall meet the conditions of having as high ionic conductance as possible and as low as possible electronic conductance, it is generally made from an electronic insulation material, such as a polypropylene film with fibrous structure. The type of electrolyte is selected depending on the characteristics of the electrode material.
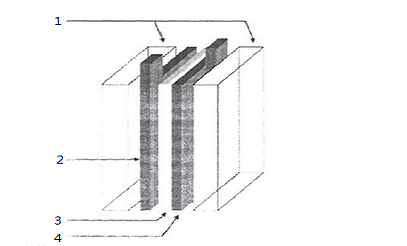
1-PTFE carrier
2 and 4: active substance on the foamed nickel collector
3-Polypropylene cell diaphragm
Supercapacitor components can vary from product to product. This is determined by the geometric structure of the supercapacitor package. For prism or square packaging, the internal structure is based on the arrangement of the internal components, that is, the internal collector is extruded from the stack of each electrode, and these collector pads will be welded to the terminal to extend the current path outside the capacitor.
For circular or cylindrical packaging, electrodes are cut into reel configuration. Finally, the electrode foil is welded to the terminal to expand the external capacitance-current path.
2) Supercapacitor Materials
At present, carbon materials are mainly used as electrode materials of surpercapacitors. In the market they are mainly activated carbon materials, because of the lower cost and high specific surface area of activated carbon, this is the characteristic that supercapacitor electrode material must have. However, the conductivity of the activated carbon is general, and the microstructure is mainly in the form of micropores, so there will be a large resistance in the electrolyte, the process of immersion of the electrode in the electrolyte will be relatively slow, either the process of storing and transferring charges. But its cost is low, it can basically meet the requirements of the market, so it is used as the main material of capacitor on the market, although other carbon materials have sound performance, but their cost is higher, so they are not commercialized. Therefore, electrode materials with good performance and low cost is the mainstream in the field of supercapacitors now. In a word, it has great significance to improve supercapacitors with superior performance and low cost, in such a situation, supercapacitors can be widely used in the market.
So far, carbon materials used to study and research electrode materials for supercapacitors include activated carbon, carbon aerogel, carbon nano-tubes, glass-carbon, graphene, carbon fiber and carbon / carbon composites. Due to the price of carbon raw material is low, surface area is large, it is suitable for mass production. However, pure carbon electrode materials do not have high specific capacitance, which need to be modified.
. Acticarbon
For activated carbon materials, different processing methods will produce different activated carbons with different specific surface areas, which can be as high as 1000 ~ 3000m2/g, and have different voids with wide pore size range, simple production process and low cost. It can be obtained from asphalt, plant shells, petroleum coke, rubber and other raw materials. And it is a commercialized electrode material for supercapacitors. Activated carbon materials can be activated by various ways, and physical activation and chemical activation are mainly used today.
2. Carbon Aerogel
Carbon aerogel is a cross-linked reticulated carbon material with porous properties. Its advantages include good conductivity, large surface area, high porosity and wide pore size distribution. Carbon aerogel is the only aerogel with high conductivity. For example, nano-carbon materials, one of carbon materials with large density span, good porosity and light weight, and meanwhile, its pore size distribution and particle size can be controlled by adjusting the process parameters.
3. Carbon Nano-tube
The carbon nano-tube is a hexagonal carbon material which is similar to graphite. A multi-layer tube, from micro perspective, its two ends are closed, and its diameter is several tens of nanometers, and the spacing of the layers is slightly larger than that of the graphite layer. For the requirement of the electrode material of the super capacitor, the carbon nano-tube material is very suitable for use as the electrode material because the structure of the carbon nanotube is hollow tube, the surface area is large, especially the carbon nano-tube with very thin walls, the specific surface area is more larger, which is very beneficial to the storage of the electric double-layer capacitor. If that carbon nano tube is made into an electrode, a special hole is also provided, the structure of holes formed between the tubes, the holes are connected to each other, and there is no plugging situation, which is very important for the flow of electrolyte when it is used as an electrode. And this kind of holes from the tube winding each other will not be too small, generally belong to the mesopore, this will make the electrode internal resistance is very low, which are required by the super capacitor electrode. At present, the research on carbon nano-tube as electrode materials for supercapacitors is mainly focused on the direct application of carbon nanotubes to supercapacitors, or the combination of carbon nanotubes and other materials as supercapacitors.
4. Activated Carbon Fiber
Activated carbon fiber (ACF) is a kind of environment-friendly material, which has better adsorption performance than activated carbon. Activated carbon fiber cloth with large specific surface area obtained from ACF has been successfully used in commercial electrode materials.
5. Graphene
Many researchers all over the world have studied graphene for a long time. Because graphene has many characteristics that other materials do not have. Its main advantages include good electrical conductivity, large surface area, low density, special thermal conductivity and optical properties, high mechanical properties and so on, these are electrode material requirements of ideal supercapacitor. There are many methods for the preparation of graphene, including tape stripping, SiC decomposition, oxidized graphite reduction and so on. The redox method is the most widely used at present. Graphene made from this method has the advantages of high yield, good quality and relatively simple process. However, this method also has disadvantages, for example, the oxide graphene after strong oxidant is not easy to be completely reduced, what’s more, the reductant may usually have highly toxic.
6. Metallic Oxide
Metal oxide material is a kind of material used on supercapacitor besides carbon material. Its storage is different from that of carbon material. It mainly adopts Faraday quasi-capacitance principle, which is much larger than double-layer capacitor. Faraday quasi-capacitance is explaining a highly reversible chemical adsorption-desorption reaction or redox reaction, which can occur at the interface between the electrodes or inside the electrodes. The capacitance values of capacitors electrode made from this material is larger than the double-layer capacitors.
7. Conductive Polymer
Conductive polymer materials are another category of electrode materials for supercapacitors, and their capacitors theory are mainly from Faraday capacitor principle. After this kind of materials are made into electrodes, a small part of the double layer capacitance occurs at the interface of the electrode solution, more is the highly reversible the redox reaction based on Faraday principle. Because conductive polymer has plasticity, it is easy to make thin layer electrode with low internal resistance and low cost.
